Our Product
Focusing on building a high-tech medical device enterprise created in China
With a global perspective, continuous innovation, and excellent service,Becoming a well-known provider of medical and health products, solutions, and services.
ABOUT US
Entering Troline
Committed to becoming a well-known provider of medical and health products, solutions, and services
Suzhou Troline Technology Co., Ltd was established in 2015. It is a high-tech company specializing in the research and development, production, and sales of medical devices, focusing on gas analysis and medical analytical instruments. We have a professional technical team that brings together experts and engineers in the field of medical devices, constantly innovating products, and developing a series of high-performance and high-precision medical devices. We always adhere to the principle ... More>>

PROFESSION
Speciality
We have a group of high-end R&D talents who have been dedicated to research and development for many years, have obtained numerous patents and core technologies, and have passed multiple medical standard tests both domestically and internationally.
COOPERATE
Cooperate
We are committed to working with all like-minded partners to bring high-quality and high-tech medical technology to more people, and to bring technology to the benefit of humanity.
QUALITY
Quality
Always regard quality as the foundation of the enterprise and maintain a sense of responsibility throughout. Ensure that each product is followed up, traceable, and strictly controlled.
INTEGRITY
Good faith
We adhere to the original intention of building people with sincerity and promoting business with trust, adhere to the business principle of "honesty and trustworthiness", and treat all partners with integrity.
News And Information
Gathering company news and understanding industry trends
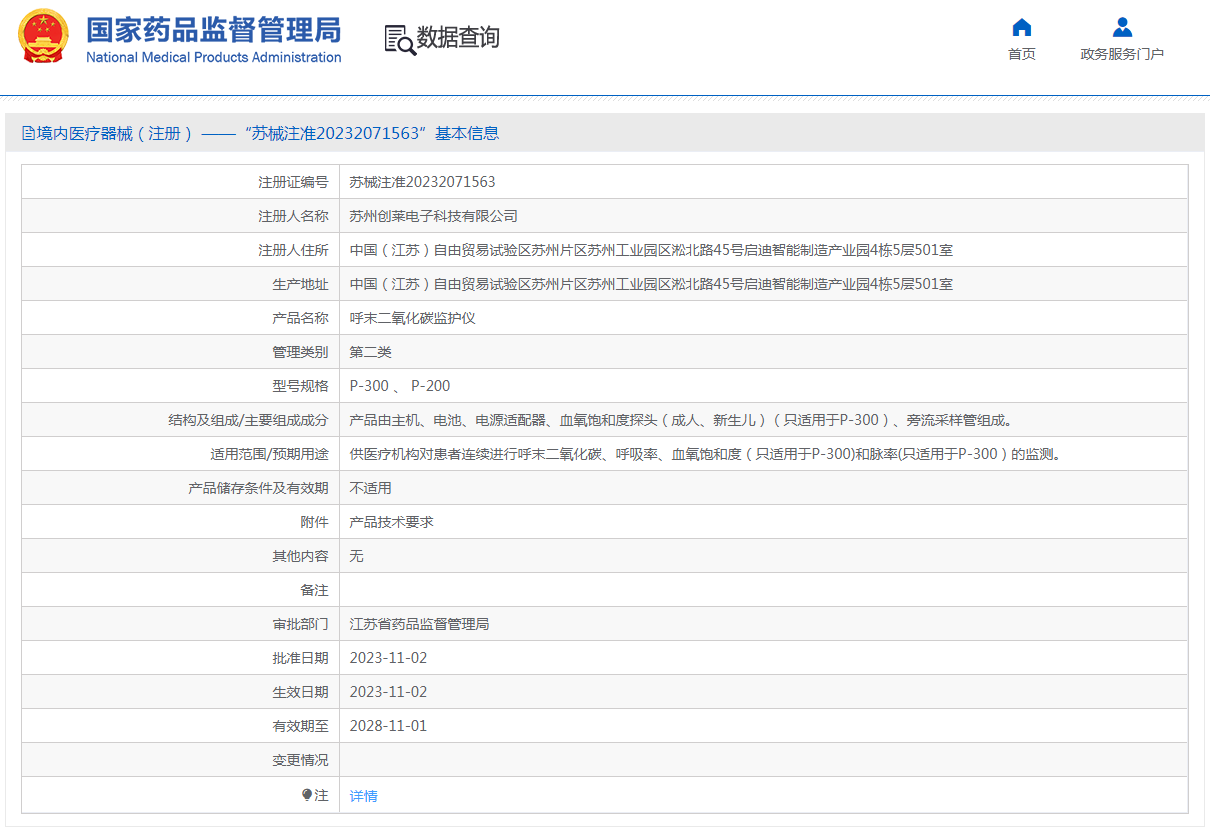
2023-12-15
Warmly celebrate the company's successful acquisition of medical device product registration certificate
Warmly celebrate the company s successful acquisition of medical device product registration certificate...
View Details >

2023-08-07
Review of the 88th CMEF Conference | First ESG and China Medical Device Development Forum
Translated from "CMEF China International Medical Device Expo " by CMEF China International Medical Device ExpoCMEF ...
View Details >

2023-12-15
The National Medical Products Administration held an on-site exchange meeting on the construction of information officer teams for grassroots drug safety coordinators
On November 28th, the National Medical Products Administration held an on-site exchange meeting on the construction of information officers for grassroots drug safety coordinators in Zhengding County, Hebei Province. ...
View Details >

2023-12-15
The National Medical Products Administration held a symposium on leading the education of young cadres and an exchange meeting on the results of the "Rooted in Grassroots" research in 2023
the National Medical Products Administration held a symposium on leading the education of young cadres and an exchange meeting on the results of the 2023 "Rooted in Grassroots" research...
View Details >
2023-12-15
Knowledge Popularization: Sample Management and Frequently Asked Questions for Medical Device Products
prefaceRegulatory requirements for sample retention of medical device products Refer to the "Quality Management...
View Details >
2023-08-07
【 Knowledge Popularization 】 Requirements for Validity Verification of Active Medical Device Products
1 Basic definitionActive medical device usage period: refers to the period during which the applicant registrant...
View Details >










